- January 9, 2025
- Argon News, Featured News
- 2 Minute Read
PLANO, Texas, January 9, 2025 /PRNewswire/ –Argon Medical Devices, a leading provider of device solutions for Interventional Radiology, Vascular Surgery, Interventional Cardiology, and Oncology procedures, announced the first patient enrollment in the CLEAN-PE study. The prospective, multicenter CLEAN-PE study aims to evaluate the safety and efficacy of the Cleaner™ Pro Thrombectomy System for removing blood clot from the lungs in patients diagnosed with pulmonary embolism (PE). CLEAN-PE is estimated to enroll over 100 patients at various hospital facilities across the United States.
The multicenter study is led by National Principal Investigator Dr. Aravinda Nanjundappa, MD, an Interventional Cardiologist at the Cleveland Clinic in Cleveland, Ohio. The first patient procedure took place at Buffalo General Medical Center in Buffalo, New York, by Interventional Cardiologist, David M. Zlotnick, MD.
PE occurs when a blood clot migrates to the lungs and restricts blood flow. This condition is life-threatening with a mortality rate up to 30%, if left untreated (1). Approximately 900,000 people in the United States alone are affected by the condition each year (2).
“We are proud to enroll the first patient in the CLEAN-PE study utilizing the Cleaner™ Pro Thrombectomy system. Unique treatment options are emerging for the management of this patient population. We are excited to evaluate a new device for patients diagnosed with pulmonary embolism.” said David M. Zlotnick, Interventional Cardiologist, Buffalo General Medical Center.
“Data indicates that pulmonary embolism mortality rates have increased in the last decade (3). We aim to help physicians improve patient outcomes by developing new solutions that enable quicker and easier PE interventions. Enrolling the first patient into the CLEAN-PE study is a monumental step forward for the solution we designed, and we are excited to discover the difference The Cleaner Vac Thrombectomy System can make for patients with this life-threatening condition.” said George Leondis, President & CEO, Argon Medical.
About Cleaner™ Pro Thrombectomy System
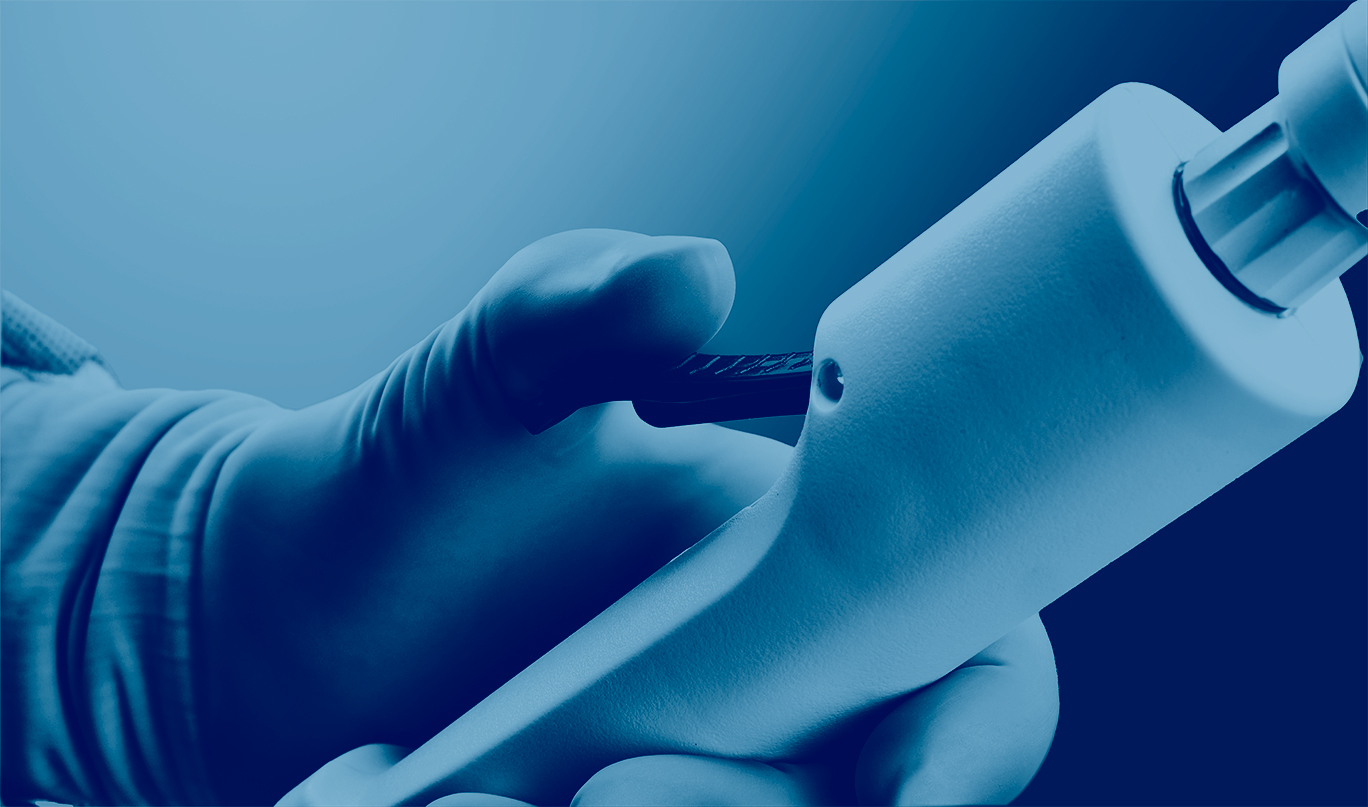
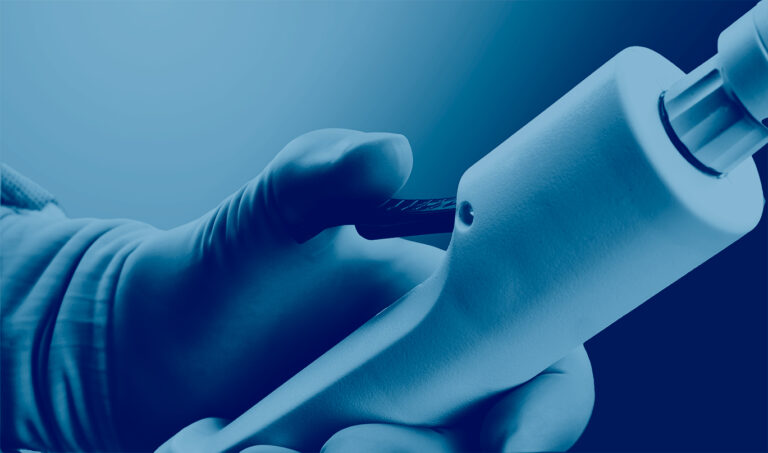
The Cleaner™ Pro Thrombectomy System is a catheter-based aspiration thrombectomy device comprised of a user-controlled handpiece, a large-bore aspiration catheter, a dilator, and a single-use aspiration pump and canister.
The system recently launched under the trade name CLEANER Vac™ Thrombectomy System for the removal of fresh, soft thrombi and emboli from the vessels of the peripheral venous vasculature.
The CLEANER Vac Thrombectomy System is not currently cleared by the FDA for use in the pulmonary vasculature for treating of pulmonary embolism.
1 Vyas V, Sankari A, Goyal A. Acute Pulmonary Embolism. [Updated 2024 Feb 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560551/
2 https://www.lung.org/lung-health-diseases/lung-disease-lookup/pulmonary-embolism/learn-about-pulmonary-embolism
3 Martin, K. A., Molsberry, R., Cuttica, M. J., Desai, K. R., Schimmel, D. R., & Khan, S. S. (2020). Time trends in pulmonary embolism mortality rates in the United States, 1999 to 2018. Journal of the American Heart Association, 9(17), e016784. https://doi.org/10.1161/JAHA.120.016784
Rx only